The Zhongke Made absorbable hemostatic microsphere project has successfully passed the ethical review of the First Affiliated Hospital of Bengbu Medical College
Release time:
2024-06-05
source:
Author:
Recently, our company's cutting-edge innovative product - absorbable hemostatic microspheres - has successfully obtained unanimous approval under the strict review of the Clinical Medical Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical College, marking another major step forward in the clinical application research of this product.
The ethics review meeting was rigorous and meticulous. The 14 members present conducted a comprehensive and in-depth review of the key content submitted by our company, including the project basis, clinical trial design scheme, product inspection report, and the company's legal qualifications. During the meeting, the committee members not only highly praised the technological innovation of the project, but also conducted forward-looking discussions on potential issues that may arise during the project implementation process from an ethical perspective, ensuring that every step of the research meets the highest ethical standards.
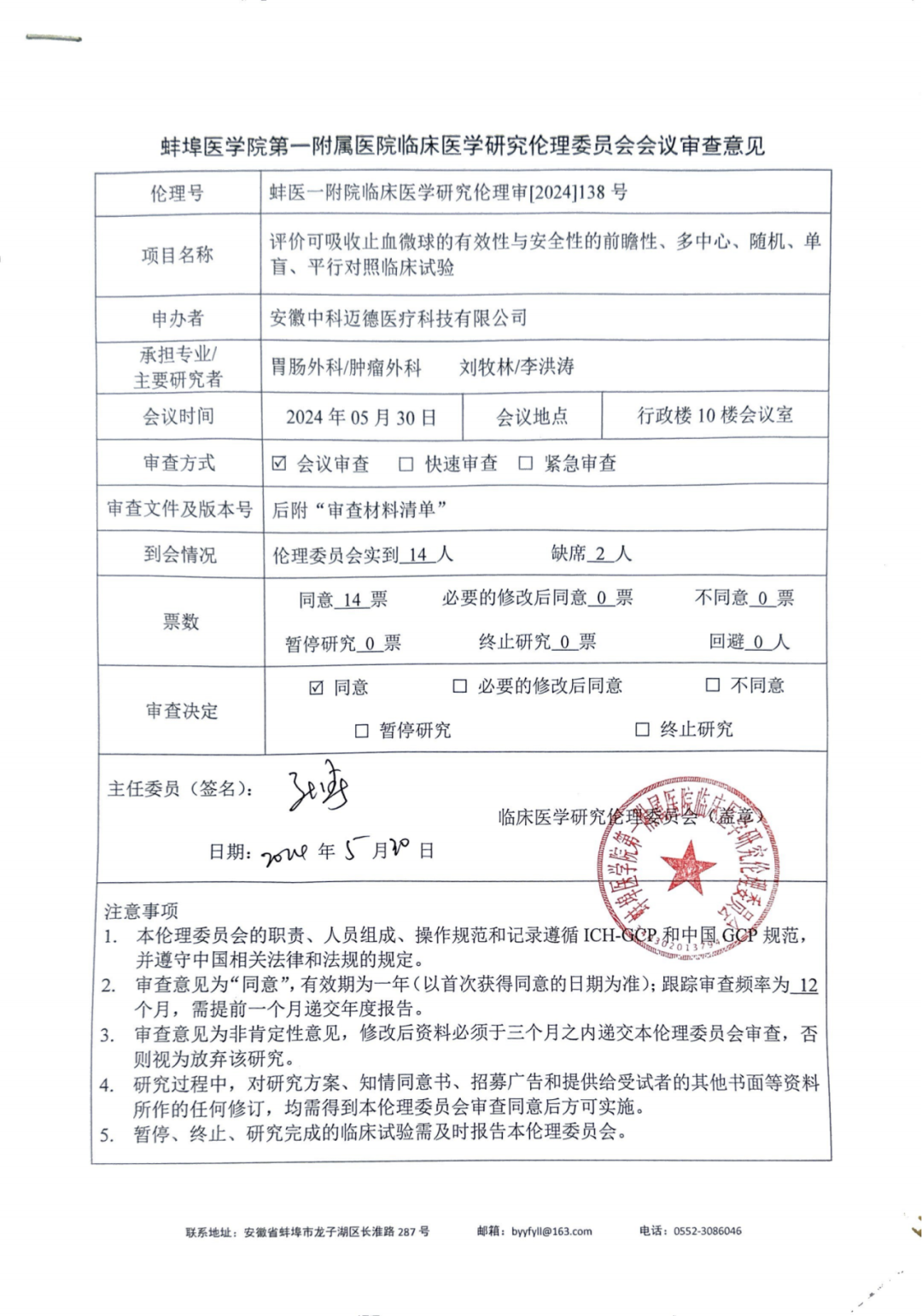
After sufficient communication and evaluation, the meeting made a decision through a vote of all members, and 14 attending committee members unanimously voted in favor. This result not only reflects the scientific rigor and innovative value of our absorbable hemostatic microsphere project, but also indicates that this technology is expected to provide a more efficient and safe solution for bleeding control in clinical surgery.

Zhongke Made absorbable hemostatic microspheres aim to achieve effective hemostasis of surgical wounds through advanced material science technology, while promoting tissue healing, reducing postoperative complications, and improving patient recovery quality. The successful passing of this ethical review has laid a solid foundation for our subsequent clinical trials, and also means that China will break the foreign monopoly in the field of absorbable hemostatic materials and overcome the bottleneck problem of key core technologies.
In the future, our company will continue to adhere to the principle of "technology leading, health first", closely follow the progress of clinical trials, ensure the safety and effectiveness of products, and strive to apply this innovative achievement to clinical practice as soon as possible, benefiting patients and contributing more to the development of medical and health care.

Conventional surgical procedures often use energy instruments such as ultrasonic knives and electric knives, as well as methods such as compression and ligation, to stop bleeding. However, if the above measures do not achieve satisfactory hemostasis results, an effective hemostatic material is urgently needed. At the same time, there is a lack of effective hemostasis methods for small incisions with deep depths, especially for bleeding at unknown bleeding points in surgical incisions.
The absorbable hemostatic microspheres developed by Anhui Zhongke Made Medical Technology Co., Ltd. can play the role of molecular sieve, rapidly absorb blood components, dehydrate blood rapidly, and make platelets, red blood cells and fibrin aggregate on the surface of particles to produce instant gel, so as to achieve immediate hemostatic effect; Simultaneously activate endogenous hemostatic factors, shorten hemostasis time, and complete hemostasis within a few minutes.
The product has passed the type inspection and the test result is qualified. In order to clarify the safety and efficacy of this product in clinical applications, according to the regulations of the National Medical Products Administration (NMPA), pre market clinical trials of this product will be conducted soon. The completion and production of Zhongkemade absorbable hemostatic microspheres means that China will break the foreign monopoly in the field of absorbable hemostatic materials, overcome the bottleneck problem of key core technologies, and meet the urgent demand for this technology product in related fields domestically.





 Message
Message
